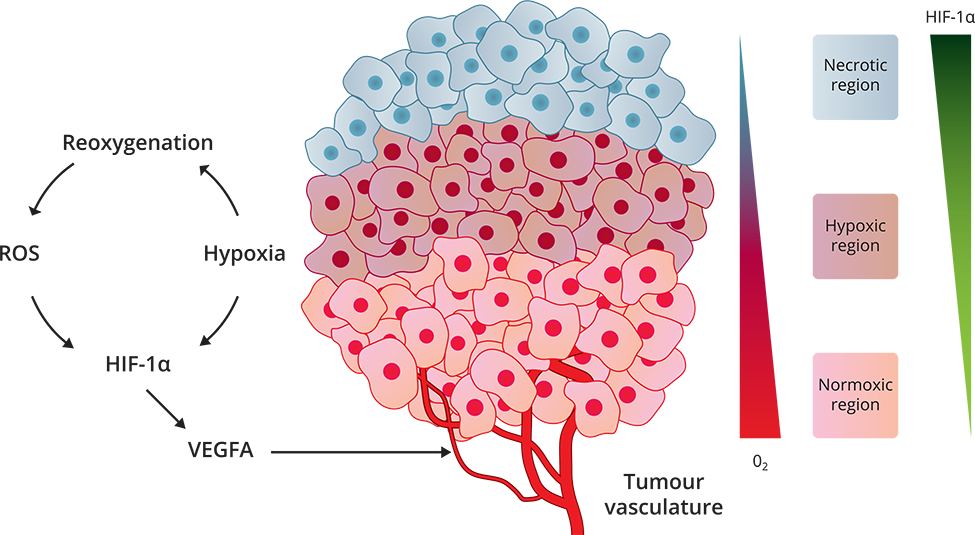
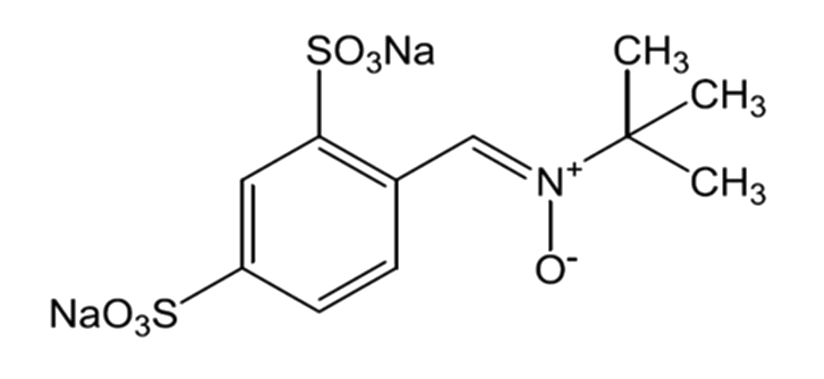
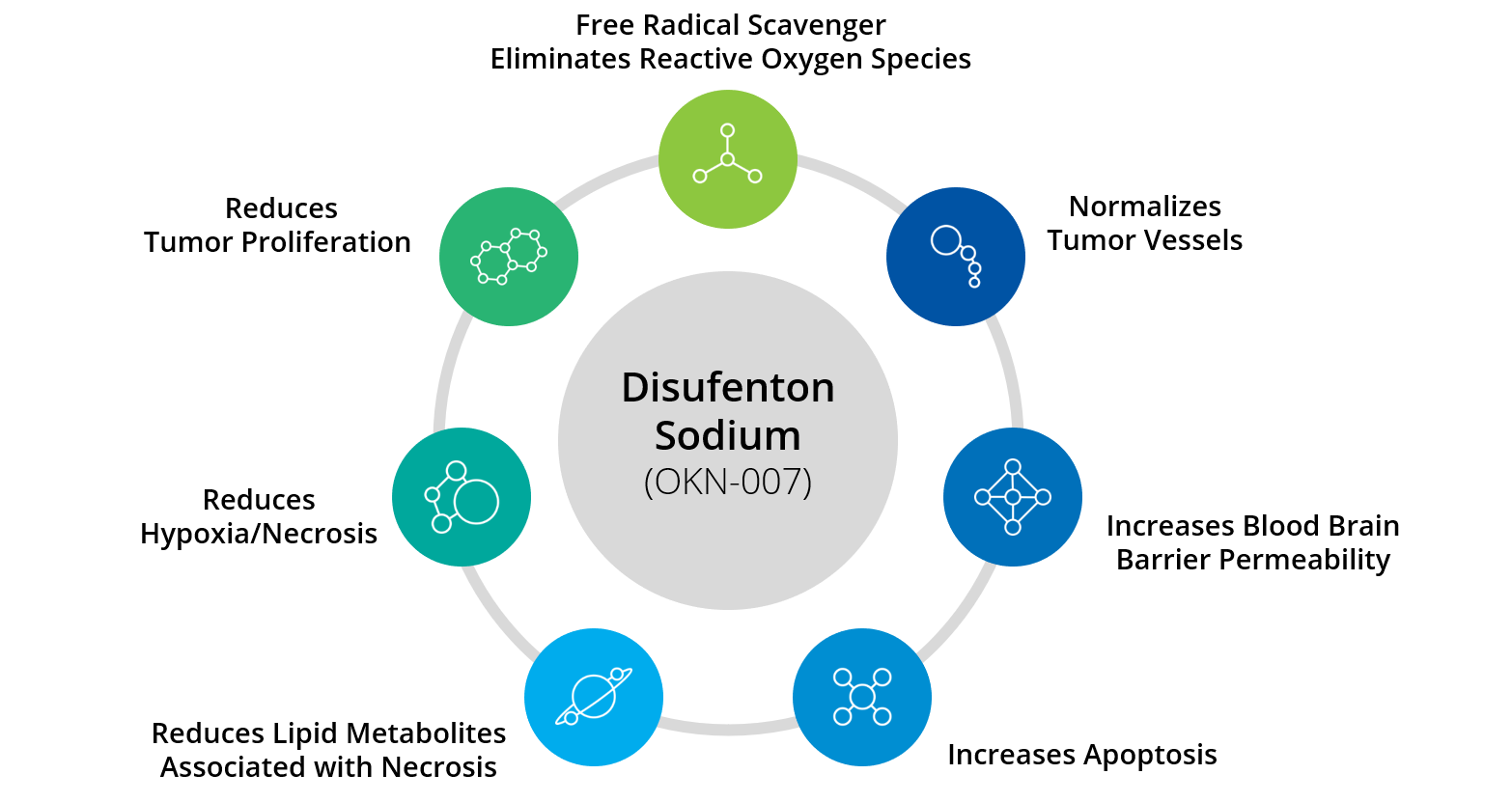
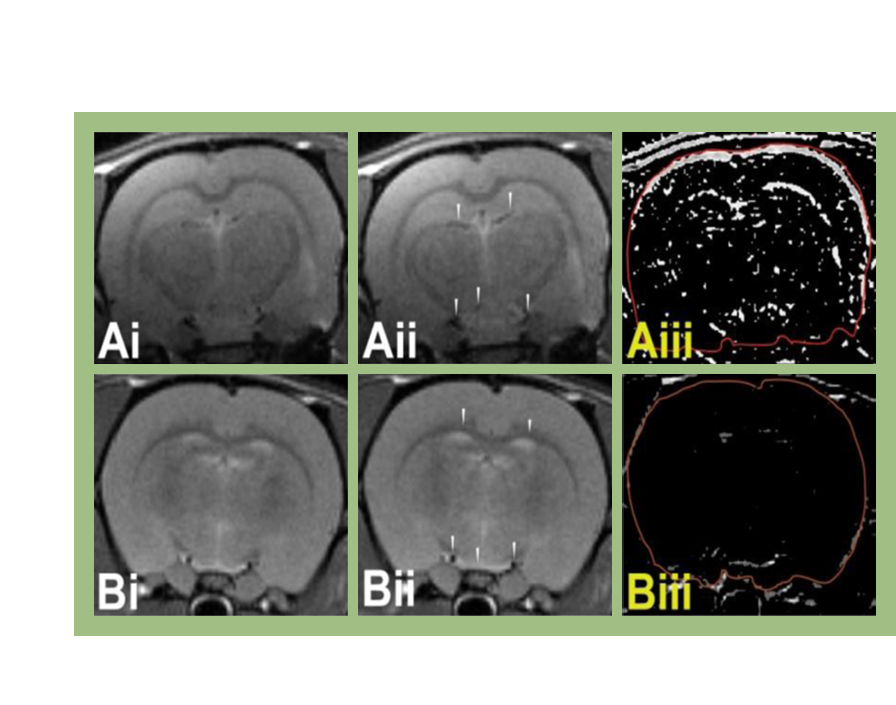
The tumor microenvironment (TME) is the environment surrounding and within a tumor. GBM tumors are hypoxic and have necrotic foci surrounded by pseudopalisades of tumor cells and microvascular hyperplasia.
Tumor hypoxia is caused by the unorganized and permeable vasculature which plays a major role in promoting aggressive and therapeutic-resistant cancers cells. This leads to rapid tumor progression and poor patient survival. Hypoxia in GBM drives tumor growth by triggering an onslaught of pathways. These responses include increased angiogenesis (blood vessel formation), tumor cell migration and invasion into the surrounding tissue, reduced apoptosis (cell death), resistance to radiation and chemotherapy, and increased tumor cell proliferation.
A major mediator of these GBM responses to hypoxia is the transcription factor HIF-1α (hypoxia-induced factor-1α). HIF-1α orchestrates GBM cellular adaptation to low oxygen by increasing angiogenesis, tumor cell survival, proliferation, migration, and invasion, and regulating pro-tumor transcription factors.