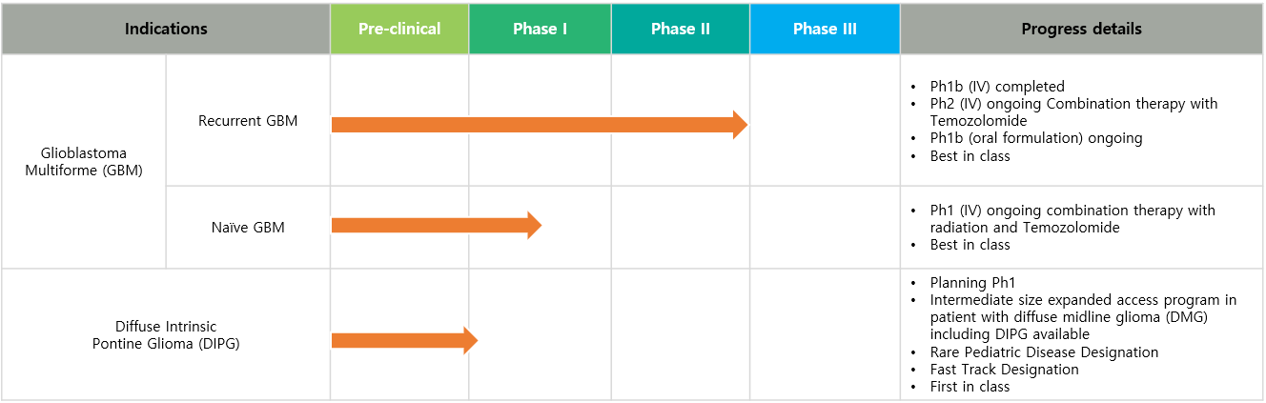
Glioblastoma Multiforme (GBM)
- Ph 1b completed with 18 recurrent GBM patients in monotherapy
- Ph 2 trial for recurrent GBM patients ongoing, a combination therapy with Temozolomide (TMZ)
- Ph 1 trial for naïve GBM patients ongoing, a combination therapy with radiation and TMZ
- Ph1b trial with oral dose of OKN-007 for recurrent GBM patients ongoing
Diffuse Intrinsic Pontine Glioma (DIPG), an ultra-rare pediatric disease
- DIPG has very grim prognosis with median survival less than 1 year. No approved therapeutic available.
- Oblato received Rare Pediatric Disease Designation from the FDA
- DIPG combination study with OKN-007 and standard of care is planned
- Oblato granted Fast Track Designation of OKN-007 for Diffuse Intrinsic Pontine Glioma from the FDA
- Oblato provides OKN-007 as an investigation drug for expanded access use in the United States for patients with diffuse midline glioma (DMG), H3 K27-altered, including DIPG.
